Studies conducted by Clinical Scholar Taia Wang, Postdoctoral Associate Jad Maamary and mentor Jeffrey V. Ravetch, which were recently published in Cell, provide exciting new information on the mechanism by which the antibody response leads to the selection of more potent antibodies over time, and open up important new translational opportunities to improve influenza vaccination. As the 2015-6 influenza season is now reaching its peak, this is welcome news!
Interactions between immune complexes and Fc receptors mediate a wide array of cellular processes that are required for maturation of protective vaccine responses. This project was designed to dissect the role of immune complex – Fc receptor interactions in the production of neutralizing anti-hemagglutinin (HA) antibodies after seasonal flu vaccination. Because B cells are selected, in part, on immune complexes that are fixed on specialized cells within the germinal center, the team hypothesized that the composition of those immune complexes might change over time after vaccination, thus providing a mechanism for directing progressive selection of B cells. In the flu system, this meant that seasonal flu vaccination might induce modulations in the composition of Fc domains on anti-HA IgGs within germinal center immune complexes; those Fc domain changes would, in turn, have a role in directing selection of B cells producing neutralizing antibodies.
To study this, they characterized changes in the structural determinants of IgG Fc domains (subclass and Fc glycan composition) on anti-HA IgGs elicited by the seasonal flu vaccine. This analysis showed something intriguing: that influenza vaccine efficacy, measured by production of neutralizing antibodies, could be predicted by the early abundance of a specific type of Fc glycoform on anti-HA IgGs. The glycoforms that correlated with influenza vaccine efficacy contained a terminal sialic acid residue, which are known to confer binding to Type II FcRs. This finding suggested that sialylated Fc domains within germinal center immune complexes might signal through B cell CD23, the only type II FcR expressed by B cells.
Next, they performed a series of experiments to determine what role, if any, CD23 signaling on B cells might play in B cell selection. The results showed that sialylated Fc glycans, which were elevated on anti-HA IgG following flu vaccination, signaled through CD23 to trigger upregulation of an inhibitory Type I FcR called FcγRIIb. Increased FcγRIIb expression, in turn, elevates the threshold of selection of B cells based on affinity of the B cell receptor (Figure 1). They found that the higher affinity anti-HA IgGs were more potent and, interestingly, provided greater breadth of protection against different influenza viruses. These results on the natural regulation of Fc domain structure during the evolution of protective vaccine responses led to identification of a mechanism driving selection of B cells within the germinal center that is dependent on CD23 signaling.
In addition, the results suggest immunization strategies involving administration of immune complexes containing sialylated Fc glycans to elicit broadly protective antibodies against influenza viruses.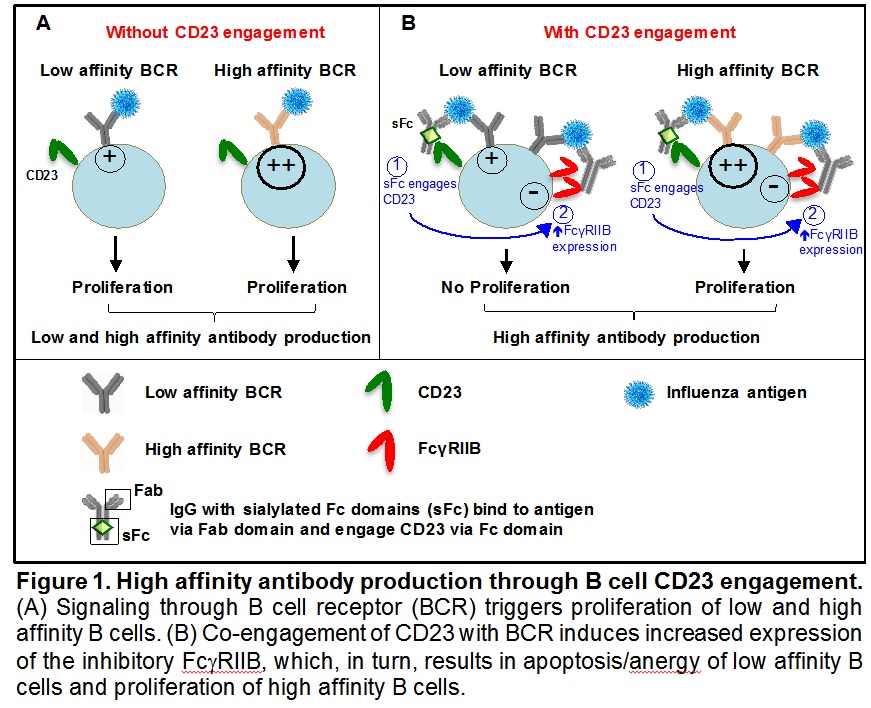
February 26, 2016
Clinical Scholar Taia Wang and Colleague Jad Maamary Discover That Anti-HA Fc Glycoforms Determines Influenza Vaccine Efficacy through Type II Fc Receptor Signaling
By Editorial Staff
