The
Rockefeller University Center for Clinical and Translational Science (CCTS)
submitted a Clinical and Translational Science Award application on September
25, 2015 to the National Center for Advancing Translational Sciences (NCATS) of
the National Institutes of Health (NIH). CCTS has been continuously funded by
the Clinical and Translational Science Award (CTSA) program since the inception
of the program in 2006, including a successful renewal application in 2011. The
grant supports the University’s high quality translational and clinical research
infrastructure that facilitates research locally, regionally and nationally and
fosters innovation in research methods, training, and career development. NCATS
has set the goal of catalyzing the development of methods and technologies that
lead to more efficient translation of biomedical discoveries into interventions
shown to improve health. To that end, the CTSA program is focused on developing
into a fully integrated research and training environment for clinical and
translational sciences that aims to dramatically improve efficiency and quality
across the translational research spectrum. The
overall vision of the Rockefeller University Center for Clinical and
Translational Science (CCTS), supported by the CTSA program, is to develop,
demonstrate, and disseminate innovative programs to achieve translational
success and to integrate these into a seamless “Learning Clinical Research
Enterprise” that uses outcome data to drive quality improvement for the benefit
of human health. To achieve this vision CCTS proposed to enhance our existing
programs and add new ones. The
Specific Aims of the proposal define the future direction of CCTS. 1. To
integrate our existing and new programs into a Translational Research
Navigation (TRN) Program that encourages, facilitates, and insures the
integrity of all human subjects research from conception to conclusion, and
that will expedite our participation in the CTSA network of multi-center
studies. 2. To integrate our existing and new programs into a Translational Workforce
Educational Program that insures that all members of the translational
workforce have the knowledge and skills required for them to perform their
functions individually and as members of diverse scientific teams. 3. To
integrate our existing and new programs into a From Discovery to
Health-Enhancing Product Program to insure that investigators have the
resources to maximize the likelihood that they can translate their novel
discoveries into products that improve human health. To achieve the vision
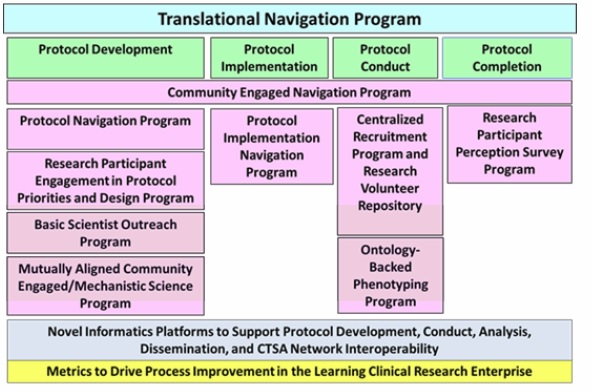
embodied in these Specific Aims, CCTS will: 1. Integrate
our Community Engaged Navigation, Protocol Navigation, Research Participant
Engagement in Protocol Priorities and Design, Basic Scientist Outreach,
Mutually Aligned Community Engaged/ Mechanistic Science, Centralized
Recruitment and Research Volunteer Repository, Ontology-Backed Phenotyping, and
Research Participant Perception programs with a new Protocol Implementation
Navigation program into an overarching TRN program under a new administrative
structure with senior leadership. TRN will be supported by an integrated
Informatics infrastructure adopting best practices and NIH and CTSA data standards.
TRN will support both local protocols and CTSA network protocols with TRN
leadership serving on the Liaisons to the Trial and Recruitment Innovation
Centers. 2. Integrate
our extensive current educational programs, including the KL2 Clinical Scholars
program, with new educational initiatives to: prepare community clinicians to
participate in research teams, enhance Clinical Research Nursing training,
provide a full range of educational experiences in translating scientific
discoveries into health-enhancing products, develop ontology-backed phenotyping
instruments, and query large electronic health record databases to test
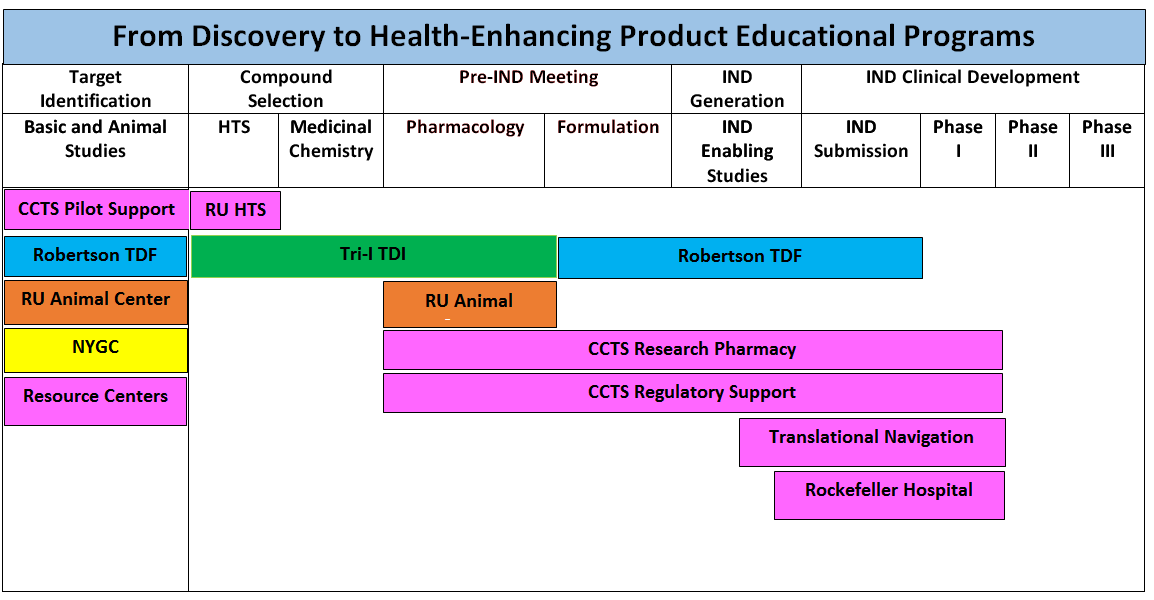
scientific hypotheses at the population level. 3. Integrate
the new Tri-Institutional Therapeutic Development Institute, which provides
access to medicinal chemists and drug project management, with the CCTS Pilot
program, the Rockefeller scientific resource centers, the New York Genome
Center, the new Robertson Therapeutic Development Fund, the TRN program, and
the CCTS Hospital to enable investigators to traverse the Valley of Death
through Phase 1/2 studies. Outcome metrics will drive performance improvement
throughout. The three diagrams below provide a pictorial summary of these
initiatives.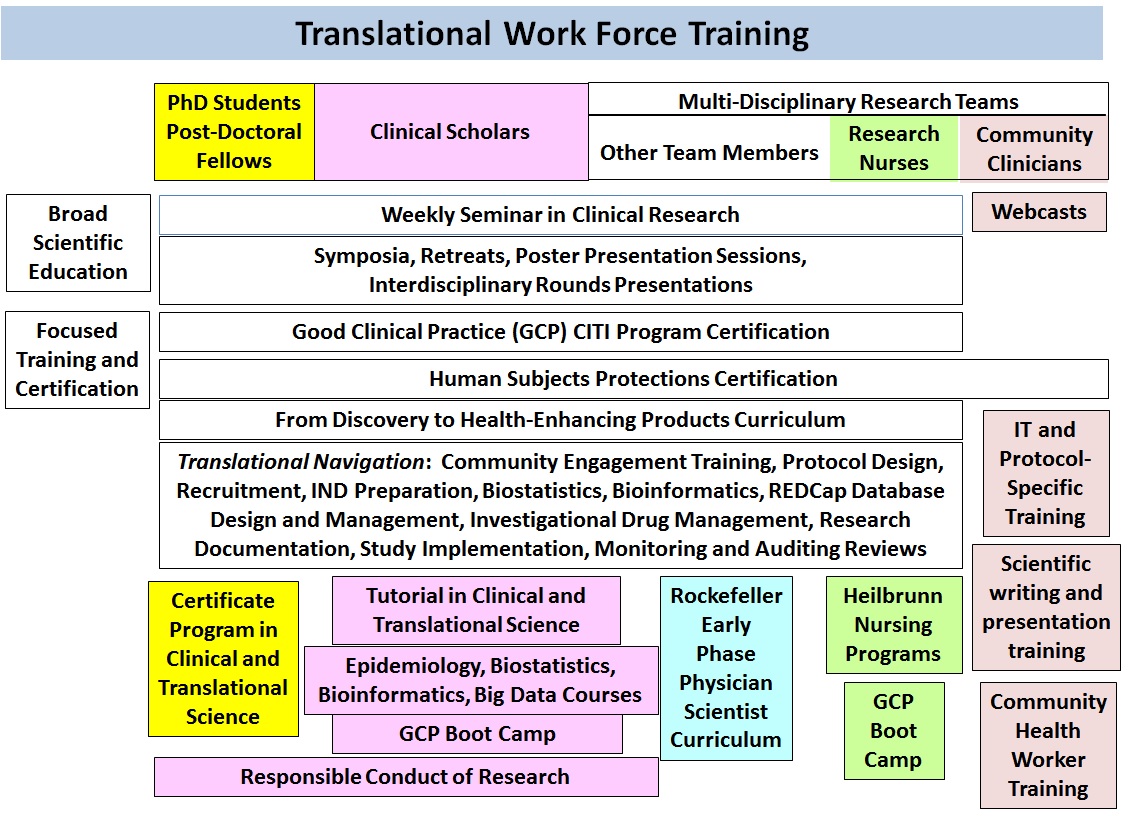
February 26, 2016
The Rockefeller University Center for Clinical and Translational Science Applies for Clinical and Translational Science Award (CTSA)
By Editorial Staff